research use only
Emricasan (IDN-6556) Caspase inhibitor
Cat.No.S7775
Chemical Structure
Molecular Weight: 569.50
Quality Control
Products Often Used Together with Emricasan (IDN-6556)
| Related Targets | Bcl-2 PD-1/PD-L1 Ferroptosis p53 Apoptosis related Synthetic Lethality STAT TNF-alpha Ras KRas |
|---|---|
| Other Caspase Inhibitors | Z-VAD-FMK Q-VD-Oph Z-DEVD-FMK Belnacasan (VX-765) Z-IETD-FMK Ac-DEVD-CHO Z-LEHD-FMK TFA Z-VAD(OH)-FMK PAC-1 Z-YVAD-FMK |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| Jurkat E6-1 | Function assay | Inhibition of Anti-Fas antibody-induced caspase-3 activity in human Jurkat E6-1 cells using Ac-DEVD-AMC substrate by fluorescence based assay, IC50 = 0.006 μM. | 29650287 | |||
| JFas | Function assay | Inhibitory concentration against JFas cells, IC50 = 0.025 μM. | 16250635 | |||
| THP-1 | Function assay | Inhibitory concentration against THP-1 cells, IC50 = 0.27 μM. | 16250635 | |||
| A673 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for A673 cells | 29435139 | |||
| Saos-2 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Saos-2 cells | 29435139 | |||
| MG 63 (6-TG R) | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for MG 63 (6-TG R) cells | 29435139 | |||
| NB1643 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for NB1643 cells | 29435139 | |||
| SK-N-MC | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SK-N-MC cells | 29435139 | |||
| Click to View More Cell Line Experimental Data | ||||||
Chemical Information, Storage & Stability
| Molecular Weight | 569.50 | Formula | C26H27F4N3O7 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 254750-02-2 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | PF 03491390, PF-03491390 | Smiles | CC(C(=O)NC(CC(=O)O)C(=O)COC1=C(C(=CC(=C1F)F)F)F)NC(=O)C(=O)NC2=CC=CC=C2C(C)(C)C | ||
Solubility
|
In vitro |
DMSO
: 100 mg/mL
(175.59 mM)
Ethanol : 25 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such as vortex, ultrasound or hot water bath can be used to aid dissolving.
Mechanism of Action
| Targets/IC50/Ki |
caspase
|
|---|---|
| In vitro |
Emricasan (IDN-6556), also called PF-03491390, is an inhibitor of activated caspases with sub- to nanomolar activity in vitro. It shows neuroprotective activity for hNPCs but does not suppress ZIKV replication.
|
| In vivo |
In the murine NASH model, stellate cell activation and hepatic fibrogenesis are attenuated by administration of Emricasan (IDN-6556), a pan-Caspase inhibitor. It decreases liver injury but not metabolic derangement in NASH and also ameliorates inflammation. This compound is currently being evaluated in phase 2 clinical trials for the reduction of hepatic injury and liver fibrosis caused by chronic HCV infection.
|
References |
|
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
| Growth inhibition assay | Cell viability |
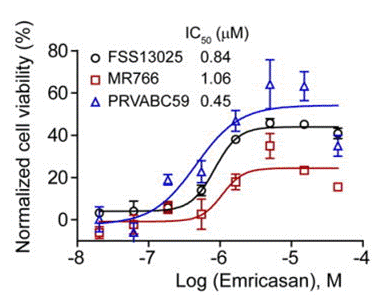
|
27571349 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT03205345 | Unknown status | Decompensated Cirrhosis |
Conatus Pharmaceuticals Inc. |
June 28 2017 | Phase 2 |
| NCT01653899 | Completed | Diabetes |
University of Alberta|Conatus Pharmaceuticals Inc. |
June 2012 | Phase 1|Phase 2 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.
