research use only
TOFA Acetyl-CoA carboxylase inhibitor
Cat.No.S6690
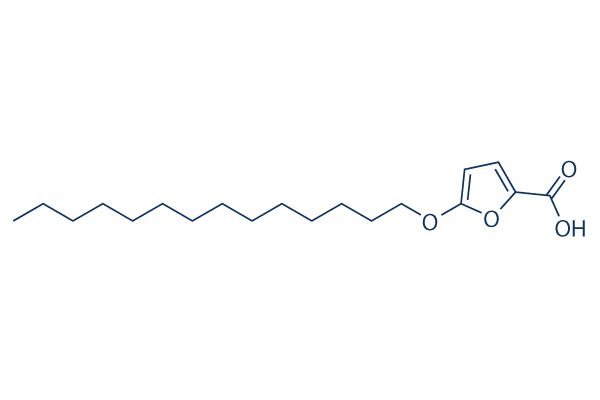
Chemical Structure
Molecular Weight: 324.45
Quality Control
| Related Targets | Dehydrogenase HSP Transferase P450 (e.g. CYP17) PDE phosphatase PPAR Vitamin Carbohydrate Metabolism Mitochondrial Metabolism |
|---|---|
| Other Acetyl-CoA carboxylase Inhibitors | Firsocostat (GS-0976, ND-630) PF-05175157 CP 640186 ND646 4-Methylsalicylic acid Oxalacetic acid PF-05221304 |
Chemical Information, Storage & Stability
| Molecular Weight | 324.45 | Formula | C19H32O4
|
Storage (From the date of receipt) | 3 years -20°C powder |
|---|---|---|---|---|---|
| CAS No. | 54857-86-2 | -- | Storage of Stock Solutions |
|
|
| Synonyms | 5-(Tetradecyloxy)-2-furoic acid, RMI14514; MDL14514 | Smiles | CCCCCCCCCCCCCCOC1=CC=C(O1)C(=O)O | ||
Solubility
|
In vitro |
DMSO
: 4 mg/mL
(12.32 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such as vortex, ultrasound or hot water bath can be used to aid dissolving.
Mechanism of Action
| Targets/IC50/Ki |
ACCA
|
References |
|---|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT05728008 | Completed | Ulcerative Colitis |
IRCCS San Raffaele |
April 5 2022 | -- |
| NCT04721808 | Completed | Arthritis Rheumatoid |
Pfizer |
January 22 2021 | -- |
| NCT03288324 | Completed | Cutaneous Lupus|Systemic Lupus Erythematosus |
Children''s Hospital Medical Center Cincinnati|Pfizer |
August 23 2017 | Phase 1|Phase 2 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.
