research use only
Omipalisib (GSK2126458) PI3K inhibitor
Cat.No.S2658
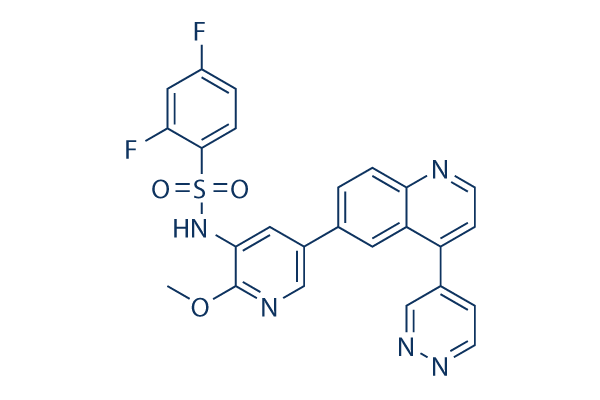
Chemical Structure
Molecular Weight: 505.5
Quality Control
| Related Targets | Akt mTOR GSK-3 ATM/ATR DNA-PK AMPK PDPK1 PTEN PP2A PDK |
|---|---|
| Other PI3K Inhibitors | GDC-0077 (Inavolisib) SAR405 Quercetin (Sophoretin) LY294002 XL147 analogue Tersolisib (STX-478) Buparlisib (BKM120) 740 Y-P (PDGFR 740Y-P) GO-203 TFA Eganelisib (IPI-549) |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| HCT116 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HCT116 cells after 72 hrs by MTT assay, IC50 = 0.01 μM. | 26819001 | ||
| Bel7404 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human Bel7404 cells after 72 hrs by MTT assay, IC50 = 0.01 μM. | 26819001 | ||
| MDA-MB-231 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MDA-MB-231 cells after 72 hrs by MTT assay, IC50 = 0.13 μM. | 26819001 | ||
| A549 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human A549 cells after 72 hrs by MTT assay, IC50 = 0.14 μM. | 27448924 | ||
| HCT116 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HCT116 cells after 72 hrs by MTT assay, IC50 = 0.154 μM. | 27448924 | ||
| U87MG | Cytotoxicity assay | 72 hrs | Cytotoxicity against human U87MG cells after 72 hrs by MTT assay, IC50 = 0.537 μM. | 27448924 | ||
| A549 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human A549 cells after 72 hrs by MTT assay, IC50 = 0.6 μM. | 26819001 | ||
| TC32 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for TC32 cells | 29435139 | |||
| U-2 OS | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for U-2 OS cells | 29435139 | |||
| A673 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for A673 cells | 29435139 | |||
| DAOY | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for DAOY cells | 29435139 | |||
| BT-37 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for BT-37 cells | 29435139 | |||
| RD | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for RD cells | 29435139 | |||
| SK-N-SH | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SK-N-SH cells | 29435139 | |||
| NB1643 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for NB1643 cells | 29435139 | |||
| OHS-50 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for OHS-50 cells | 29435139 | |||
| LAN-5 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for LAN-5 cells | 29435139 | |||
| NB-EBc1 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for NB-EBc1 cells | 29435139 | |||
| SK-N-SH | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for SK-N-SH cells | 29435139 | |||
| Rh41 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Rh41 cells | 29435139 | |||
| A673 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for A673 cells) | 29435139 | |||
| BT-37 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for BT-37 cells | 29435139 | |||
| MG 63 (6-TG R) | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for MG 63 (6-TG R) cells | 29435139 | |||
| Rh30 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Rh30 cells | 29435139 | |||
| Rh30 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for Rh30 cells | 29435139 | |||
| OHS-50 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for OHS-50 cells | 29435139 | |||
| Rh41 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for Rh41 cells | 29435139 | |||
| SJ-GBM2 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SJ-GBM2 cells | 29435139 | |||
| SK-N-MC | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SK-N-MC cells | 29435139 | |||
| NB-EBc1 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for NB-EBc1 cells | 29435139 | |||
| LAN-5 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for LAN-5 cells | 29435139 | |||
| Rh18 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Rh18 cells | 29435139 | |||
| NB1643 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for NB1643 cells | 29435139 | |||
| SK-N-MC | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for SK-N-MC cells | 29435139 | |||
| TC32 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for TC32 cells | 29435139 | |||
| Rh18 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for Rh18 cells | 29435139 | |||
| Saos-2 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for Saos-2 cells | 29435139 | |||
| Click to View More Cell Line Experimental Data | ||||||
Chemical Information, Storage & Stability
| Molecular Weight | 505.5 | Formula | C25H17F2N5O3S |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 1086062-66-9 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | GSK458 | Smiles | COC1=C(C=C(C=N1)C2=CC3=C(C=CN=C3C=C2)C4=CN=NC=C4)NS(=O)(=O)C5=C(C=C(C=C5)F)F | ||
Solubility
|
In vitro |
DMSO
: 101 mg/mL
(199.8 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such as vortex, ultrasound or hot water bath can be used to aid dissolving.
Mechanism of Action
| Targets/IC50/Ki |
p110α
(Cell-free assay) 0.019 nM(Ki)
p110δ
(Cell-free assay) 0.024 nM(Ki)
p110γ
(Cell-free assay) 0.06 nM(Ki)
p110β
(Cell-free assay) 0.13 nM(Ki)
mTORC1
(Cell-free assay) 0.18 nM(Ki)
mTORC2
(Cell-free assay) 0.3 nM(Ki)
|
|---|---|
| In vitro |
Omipalisib (GSK2126458) potently inhibits the activity of common activating mutants of p110α (E542K, E545K, and H1047R) found in human cancer with Ki of 8 pM, 8 pM and 9 pM, respectively. This compound causes a significant reduction in the levels of pAkt-S473 with remarkable potency in T47D and BT474 cells with IC50 of 0.41 nM and 0.18 nM, respectively. Furthermore, it leads to a G1 cell cycle arrest and produces the inhibitory effect on cell proliferation in a large panel of cell lines, including T47D and BT474 breast cancer lines with IC50 of 3 nM and 2.4 nM, respectively.
|
| Kinase Assay |
HTRF In vitro Profiling Assays for PI3K Inhibition
|
|
Compounds are serially diluted (3-fold in 100% DMSO) across a 384-well polypropylene mother plate from column 1 to column 12 and column 13 to column 24, to yield 11 concentrations for Omipalisib (GSK2126458). Columns 6 and 18 contain only DMSO. Once titrations are made, 0.05μL is transferred to a 384-well low-volume assay plate. This assay plate contains three pharmacological controls (known PI3K inhibitors) and 3 assay controls: (1) Enzyme without inhibitor; (2) Buffer minus enzyme, and (3) Buffer minus enzyme plus native PIP3. DMSO is stamped into all wells of columns 6 and 18. PIP3 is added at 40 μM in 1X Reaction buffer (1μL of 200 μM PIP3) to alternating rows of column 18 (wells 18 B, D, F, H, J, L, N, P). The no-enzyme control reactions are run in wells 18 A, C, E, G, I, K, M, O (0.1μL of 100% DMSO). The PI3-Kinase profiling assay is optimized using the HTRF kit. The assay kit contains seven reagents: 1) 4X Reaction Buffer; 2) native PIP2 (substrate); 3) Stop A (EDTA); 4) Stop B (Biotin-PIP3); 5) Detection Mix A (Streptavidin-APC); 6) Detection Mix B (Eu-labelled Anti-GST plus GST-tagged PHdomain); 7) Detection Mix C (KF). PI3Kinase Reaction Buffer is prepared by diluting the stock 1:4 with de-ionized water. Freshly prepared DTT is added at a final concentration of 5 mM on the day of use. Enzyme addition and compound pre-incubation are initiated by the addition of 2.5μL of PI3K (at twice its final concentration) in 1X reaction buffer to all wells using a Multidrop Combi. Plates are incubated at room temperature for 15 minutes. Reactions are initiated by addition of 2.5μL of 2X substrate solution (PIP2 and ATP in 1X reaction buffer) using a Multidrop Combi. Plates are incubated at room temperature for one hour. Reactions are quenched by the addition of 2.5μL of stop solution (Stop A and Stop B pre-mixed at a ratio of 5:1, respectively) to all wells using the Multidrop Combi. The quenched reactions are then processed to detect product formation by adding 2.5μL of Detection Solution to all wells using the Mulitdrop Combi (Detection mix C, Detection mix A, and Detection mix B combined together in an 18:1:1 ratio, i.e.: for a 6000 μL total volume, mix 5400 μL Detection mix C, 300μL Detection mix A, and 300 μL Detection mix B. Note: this solution should be prepared 2 hours prior to use). Following a one hour incubation in the dark, the HTRF signal is measured on the Envision plate reader set for 330nm excitation and dual emission detection at 620nm (Eu) and 665nm (APC).
|
|
| In vivo |
In a BT474 human tumour xenograft model, Omipalisib (GSK2126458) treatment results in a dose-dependent reduction in pAkt-S473 levels, and exhibited dose-dependent tumour growth inhibition at a low dose of 300 μg/kg. Besides, it shows low blood clearance and good oral bioavailability in four preclinical species (mouse, rat, dog, and monkey).
|
References |
|
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
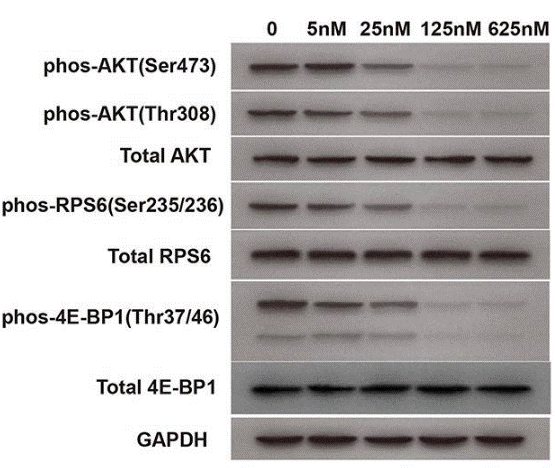
| Western blot | p-AKT / AKT / p-RPS6 / RPS6 / p-4E-BP1 / 4E-BP1 |
|
31069214 |
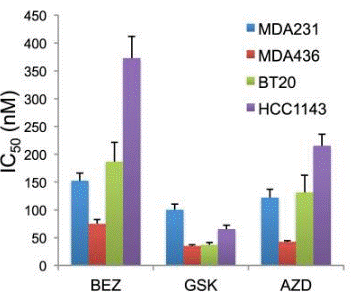
| Growth inhibition assay | IC50 |
|
26148118 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT01725139 | Completed | Idiopathic Pulmonary Fibrosis |
GlaxoSmithKline |
March 8 2013 | Phase 1 |
| NCT01248858 | Terminated | Cancer |
GlaxoSmithKline |
December 3 2010 | Phase 1 |
| NCT00972686 | Completed | Solid Tumours |
GlaxoSmithKline |
August 31 2009 | Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.
