XL092 (JUN04542) is an ATP-competitive inhibitor of multiple RTKs including MET, VEGFR2, AXL and MER, with IC50 values of 15 nM, 1.6 nM, 3.4 nM, and 7.2 nM in cell-based assays, respectively.
research use only
XL092 c-Met inhibitor
Cat.No.E0142
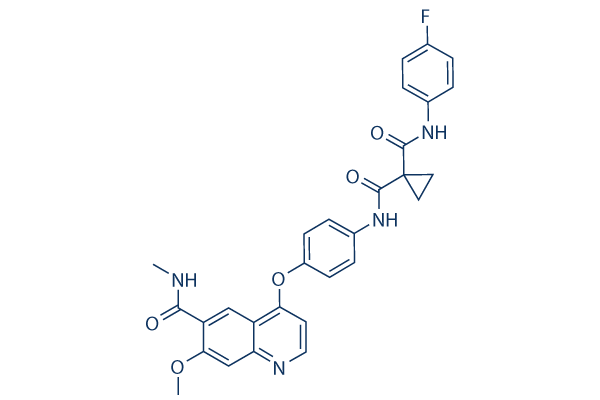
Chemical Structure
Molecular Weight: 528.53
Quality Control
| Related Targets | EGFR VEGFR PDGFR FGFR Src MEK CSF-1R FLT3 HER2 c-Kit |
|---|---|
| Other c-Met Inhibitors | Tepotinib Dihexa SGX-523 PHA-665752 Foretinib SU11274 BMS-777607 JNJ-38877605 Tivantinib PF-04217903 |
Chemical Information, Storage & Stability
| Molecular Weight | 528.53 | Formula | C29H25FN4O5 |
Storage (From the date of receipt) | 3 years -20°C powder |
|---|---|---|---|---|---|
| CAS No. | 2367004-54-2 | -- | Storage of Stock Solutions |
|
|
| Synonyms | JUN04542 | Smiles | CNC(=O)C1=CC2=C(OC3=CC=C(NC(=O)C4(CC4)C(=O)NC5=CC=C(F)C=C5)C=C3)C=CN=C2C=C1OC | ||
Solubility
|
In vitro |
DMSO
: 100 mg/mL
(189.2 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such as vortex, ultrasound or hot water bath can be used to aid dissolving.
Mechanism of Action
| Targets/IC50/Ki |
VEGFR2
(in cell-based assay) 1.6 nM
AXL
(in cell-based assay) 3.4 nM
MER
(in cell-based assay) 7.2 nM
MET
(in cell-based assay) 15 nM
|
References |
|---|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT03845166 | Active not recruiting | Neoplasm Malignant|Renal Cell Carcinoma|Hormone Receptor Positive Breast Carcinoma|Metastatic Castration-resistant Prostate Cancer|Colorectal Cancer |
Exelixis |
March 20 2019 | Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.
