research use only
Lucitanib (E3810) hydrochloride VEGFR inhibitor
Cat.No.S7647
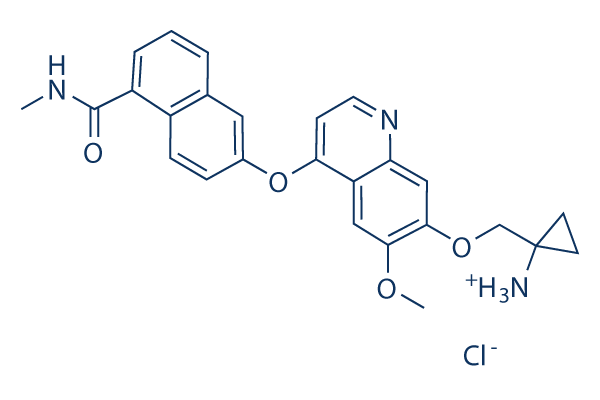
Chemical Structure
Molecular Weight: 479.96
Quality Control
| Related Targets | EGFR PDGFR FGFR c-Met Src MEK CSF-1R FLT3 HER2 c-Kit |
|---|---|
| Other VEGFR Inhibitors | SAR131675 SU 5402 Cediranib (AZD2171) Vatalanib (PTK787) 2HCl Anlotinib (AL3818) Dihydrochloride Linifanib (ABT-869) Apatinib (YN968D1) Apatinib (YN968D1) mesylate Ki8751 ZM 323881 HCl |
Chemical Information, Storage & Stability
| Molecular Weight | 479.96 | Formula | C26H26ClN3O4 |
Storage (From the date of receipt) | 3 years -20°C powder |
|---|---|---|---|---|---|
| CAS No. | 1058137-23-7 | -- | Storage of Stock Solutions |
|
|
| Synonyms | AL3810 | Smiles | [Cl-].CNC(=O)C1=CC=CC2=C1C=CC(=C2)OC3=CC=NC4=CC(=C(OC)C=C34)OCC5([NH3+])CC5 | ||
Solubility
|
In vitro |
DMSO
: 59 mg/mL
(122.92 mM)
Water : 59 mg/mL Ethanol : 30 mg/mL |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such as vortex, ultrasound or hot water bath can be used to aid dissolving.
Mechanism of Action
| Targets/IC50/Ki |
VEGFR1
(Cell-free assay) 7 nM
VEGFR3
(Cell-free assay) 10 nM
FGFR1
(Cell-free assay) 17.5 nM
VEGFR2
(Cell-free assay) 25 nM
FGFR2
(Cell-free assay) 82.5 nM
|
|---|---|
| In vitro |
In vitro Lucitanib (E3810) inhibits the VEGF- and bFGF-dependent proliferation and the signaling transduction pathways elicited by VEGF and bFGF ligands binding to their cognate receptors in HUVEC cells in the nanomolar range. |
| In vivo |
In vivo 7 days of treatment with Lucitanib (E3810) completely inhibites the FGF-induced angiogenesis in an implanted Matrigel plug in mice; Lucitanib (E3810) treatment significantly reduces tumor vessel density in treated tumors (as assessed by the decrease in CD31 staining), increasing in the percentage of tumor necrosis and changing the composition of tumor stroma (with a decrease in collagen IV content). |
References |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT04042116 | Suspended | Advanced Solid Tumor|Gynecologic Cancer |
Clovis Oncology Inc.|Bristol-Myers Squibb|European Network of Gynaecological Oncological Trial Groups (ENGOT) |
July 29 2019 | Phase 1|Phase 2 |
| NCT02747797 | Withdrawn | Advanced Cancer |
Teresa Helsten MD|Clovis Oncology Inc.|University of California San Diego |
April 2017 | Phase 2 |
| NCT02202746 | Terminated | Breast Cancer|Metastatic Breast Cancer|MBC|HER2 Positive|HER2|Estrogen Receptor Positive|ER|Triple Negative |
Clovis Oncology Inc. |
September 9 2014 | Phase 2 |
| NCT02109016 | Terminated | Non-Small Cell Lung Cancer|Squamous Non-Small Cell Lung Cancer|NSCLC|Small Cell Lung Cancer|SCLC|Lung Cancer|Advanced Lung Cancer|Metastatic Lung Cancer|Stage IV Lung Cancer |
Clovis Oncology Inc. |
April 2014 | Phase 2 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.
