research use only
GSK923295 Kinesin inhibitor
Cat.No.S7090
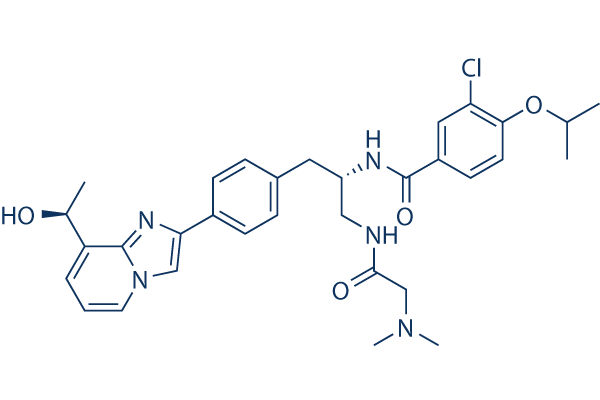
Chemical Structure
Molecular Weight: 592.13
Quality Control
| Related Targets | Akt Wnt/beta-catenin PKC HSP ROCK Microtubule Associated Integrin Bcr-Abl Actin FAK |
|---|---|
| Other Kinesin Inhibitors | Ispinesib (SB-715992) SB743921 HCl Monastrol ARQ 621 K 858 BTB-1 VLS-1488(KIF18A-IN-6 ) H-Cys(Trt)-OH Filanesib hydrochloride GW406108X |
Chemical Information, Storage & Stability
| Molecular Weight | 592.13 | Formula | C32H38ClN5O4 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 1088965-37-0 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | N/A | Smiles | CC(C)OC1=C(C=C(C=C1)C(=O)NC(CC2=CC=C(C=C2)C3=CN4C=CC=C(C4=N3)C(C)O)CNC(=O)CN(C)C)Cl | ||
Solubility
|
In vitro |
DMSO
: 100 mg/mL
(168.88 mM)
Ethanol : 100 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such as vortex, ultrasound or hot water bath can be used to aid dissolving.
Mechanism of Action
| Features |
First potent, CENP-E-selective inhibitor that has been tested in Phase I clinical trials for treatment of Refractory Cancers.
|
|---|---|
| Targets/IC50/Ki |
CENP-E
(Cell-free assay) 3.2 nM(Ki)
|
| In vitro |
GSK923295 is the first potent and selective inhibitor of the mitotic kinesin centromere-associated protein-E (CENP-E). This compound is uncompetitive with both ATP and microtubules (MT), inhibiting CENP-E MT-stimulated ATPase activity with a Ki of 3.2 nM, highly selective over other kinesins. It inhibits release of inorganic phosphate and stabilises CENP-E motor domain interaction with microtubules, reduces the rate of ATP-promoted dissociation of CENP-E from MT (koff, MT) by more than 50-fold. This chemical causes failure of metaphase chromosome alignment and induces mitotic arrest. It is a potent inhibitor of tumour cell growth, with an average GI50 of 253 nM and a median GI50 of 32 nM for 237 tumour cell lines. This compound inhibits tumour cell growth more effectively when mitogen-activated protein kinase (MEK/ERK) signalling is also inhibited. |
| Kinase Assay |
Enzymology
|
|
Kinesin motor domains are expressed in Escherichia coli BL21(DE3) and purified. CENP-E proteins includes residues 2–340 with a carboxyl-terminal 6-his tag. All studies using MT are conducted in PEM25 buffer [25mM PipesK+ (pH 6.8), 2mM MgCl2, 1mM EGTA] . The IC50 for steady-state inhibition is determined at 500 μM ATP, 5 μM MT, and 1 nM CENP-E in PEM25 buffer.
|
|
| In vivo |
GSK923295 produces clear increases in the abundance of mitotic figures and scattered apoptotic bodies in tumours. This compound causes a dose-dependent increase in the ratio of 4n to 2n nuclei. It exhibits robust, dose-dependent antitumour activity against Colo205 xenografts, including partial and complete regressions at the 125 mg/kg dose. It demonstrates significant antitumour activity against solid tumour models, inducing CRs in Ewing sarcoma, rhabdoid, and rhabdomyosarcoma xenografts, may be a valuable therapeutic target in paediatric cancer. |
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT00504790 | Completed | Cancer |
GlaxoSmithKline |
June 25 2007 | Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.
