research use only
Dapivirine (TMC120) Reverse Transcriptase inhibitor
Cat.No.S2914
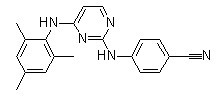
Chemical Structure
Molecular Weight: 329.4
Quality Control
| Related Targets | Integrase Bacterial Antibiotics Anti-infection Fungal Antiviral COVID-19 Parasite HIV HCV Protease |
|---|---|
| Other Reverse Transcriptase Inhibitors | Fangchinoline Salicylanilide 3'-Fluoro-3'-deoxythymidine (Alovudine) Ulonivirine Lersivirine (UK-453061) Bifendate 4-Chloro-2-(trifluoroacetyl)aniline hydrochloride |
Chemical Information, Storage & Stability
| Molecular Weight | 329.4 | Formula | C20H19N5 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 244767-67-7 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | N/A | Smiles | CC1=CC(=C(C(=C1)C)NC2=NC(=NC=C2)NC3=CC=C(C=C3)C#N)C | ||
Solubility
|
In vitro |
DMSO
: 34 mg/mL
(103.21 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such as vortex, ultrasound or hot water bath can be used to aid dissolving.
Mechanism of Action
| Targets/IC50/Ki |
HIV reverse transcriptase
24 nM
|
|---|---|
| In vitro |
Dapivirine (TMC120) prevents HIV-induced syncytium formation in the nanomolar range and shows a low cytostatic activity. It apparently blocks HIV-1 infection in the primary cultures at a 10 nM concentration, but secondary cultures reveal that a 100 nM concentration is needed to completely prevent proviral integration. This compound is well tolerated by epithelial cells, T cells, macrophages, and cervical tissue explants with CC50 (50% cytotoxic concentration) of 10 μM to 20 μM. It potently inhibits infection by both X4- and R5-utilizing HIV-1 strains with IC50 of 1.46 nM in cell-based assays. Dapivirine potently inhibits HIV-1BaL infection of human ectocervical explant tissue in a dose-dependent manner, as evaluated by the reduction in both p24 release and provirus content in cultured explants. It inhibits the transmission of virus to permissive T cells in a dose-dependent manner, with an IC50 of 0.1 nM. This compound results in significant inhibition of HIV infection when explants are challenged with virus immediately with IC90 of 100 nM. It is also able to inhibit viral dissemination by migratory cells.
|
| In vivo |
Dapivirine (TMC120)-containing gel at vaginal level inhibits cell-associated HIV infection in mice. More placebo (7 of 12) than this compound (3 of 24) gel users had positive vaginal swab results, with white blood cells being the most common finding. It results in Cmax of 715 pg/mL, AUC of 15 ng×h/mL and T1/2 of 89.87 hours in plasma after 14 days post-dose. Mean concentrations of this compound (0.05%) in vaginal fluids collected at the introitus, mid vagina, and cervix are in the range of 62-265 μg/g on day 1.
|
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT05416021 | Active not recruiting | HIV Infections |
International Partnership for Microbicides Inc. |
August 1 2022 | Phase 1 |
| NCT03593655 | Completed | HIV Infections |
National Institute of Allergy and Infectious Diseases (NIAID) |
January 14 2019 | Phase 2 |
| NCT03393468 | Completed | HIV Infections |
National Institute of Allergy and Infectious Diseases (NIAID) |
May 10 2018 | Phase 1 |
| NCT03239483 | Completed | HIV Infections |
National Institute of Allergy and Infectious Diseases (NIAID) |
October 26 2017 | Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.
