research use only
Cilengitide trifluoroacetate Integrin inhibitor
Cat.No.S7077
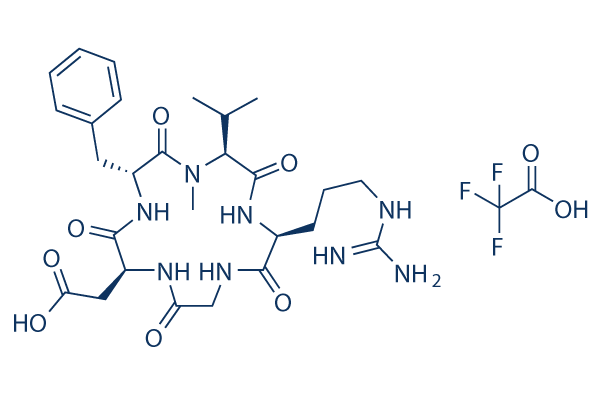
Chemical Structure
Molecular Weight: 702.68
Quality Control
| Related Targets | Akt Wnt/beta-catenin PKC HSP ROCK Microtubule Associated Bcr-Abl Actin FAK Kinesin |
|---|---|
| Other Integrin Inhibitors | SB273005 Cilengitide (EMD 121974) RGD peptide (Arg-Gly-Asp) ATN-161 Cyclo(-RGDfK) TFA Cyclo(RGDyK) TFA Leukadherin-1 A-205804 Pyrintegrin RGD peptide (GRGDNP) |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| G28 | Function Assay | 50 μg/ml | 30/60/120 min | inhibits phosphorylation of FAK, Src and Akt | 19114005 | |
| HMEC-1 | Function Assay | 20/40/60 μg/ml | inhibits FAK and Src | 19114005 | ||
| G44 | Apoptosis Assay | 1/5/50 μg/ml | 24 h | induces apoptosis | 19114005 | |
| G28 | Apoptosis Assay | 1/5/50 μg/ml | 24 h | induces apoptosis | 19114005 | |
| G44 | Proliferation Assay | 1/5/50 μg/ml | 24/48/72 h | inhibits proliferation in a dose dependent manner | 19114005 | |
| G28 | Proliferation Assay | 1/5/50 μg/ml | 24/48/72 h | inhibits proliferation in a dose dependent manner | 19114005 | |
| HMEC-1 | Apoptosis Assay | 1/5/50 μg/ml | 24 h | induces apoptosis | 19114005 | |
| HMEC-1 | Proliferation Assay | 1/5/50 μg/ml | 24/48/72 h | inhibits proliferation in a dose dependent manner | 19114005 | |
| HMEC-1 | Function Assay | 1/5/50 μg/ml | 24 h | induces a dose dependent detachment | 19114005 | |
| LNT-229 | Function Assay | 0.1/1/10 μM | 24 h | impairs the adhesion of cells to vitronectin in a dose dependent manner | 19221171 | |
| T98G | Function Assay | 0.1/1/10 μM | 24 h | impairs the adhesion of cells to vitronectin in a dose dependent manner | 19221171 | |
| LN-18 | Function Assay | 0.1/1/10 μM | 24 h | impairs the adhesion of cells to vitronectin in a dose dependent manner | 19221171 | |
| LN-308 | Function Assay | 0.1/1/10 μM | 24 h | impairs the adhesion of cells to vitronectin in a dose dependent manner | 19221171 | |
| U87MG | Function Assay | 0.1/1/10 μM | 24 h | impairs the adhesion of cells to vitronectin in a dose dependent manner | 19221171 | |
| U87 | Function Assay | 0-25 μg/mL | 12 h | induces autophagy dose dependently | 21788343 | |
| U251 | Function Assay | 0-25 μg/mL | 12 h | induces autophagy dose dependently | 21788343 | |
| U87 | Apoptosis Assay | 25 μg/mL | 24/48 h | induces apoptosis at 48 h significantly | 21788343 | |
| U251 | Apoptosis Assay | 25 μg/mL | 24/48 h | induces apoptosis at 48 h significantly | 21788343 | |
| U87 | Growth Inhibition Assay | 0-25 μg/mL | 0-48 h | inhibits cell growth in dose and time dependent manner | 21788343 | |
| U251 | Growth Inhibition Assay | 0-25 μg/mL | 0-48 h | inhibits cell growth in dose and time dependent manner | 21788343 | |
| U251MG | Apoptosis Assay | 1 µM | 48 h | induces apoptosis | 23354807 | |
| U87MG | Apoptosis Assay | 1 µM | 48 h | induces apoptosis | 23354807 | |
| U251MG | Growth Inhibition Assay | 0-25 μM | 24/48 h | inhibits cell growth in dose and time dependent manner | 23354807 | |
| U87MG | Growth Inhibition Assay | 0-25 μM | 24/48 h | inhibits cell growth in dose and time dependent manner | 23354807 | |
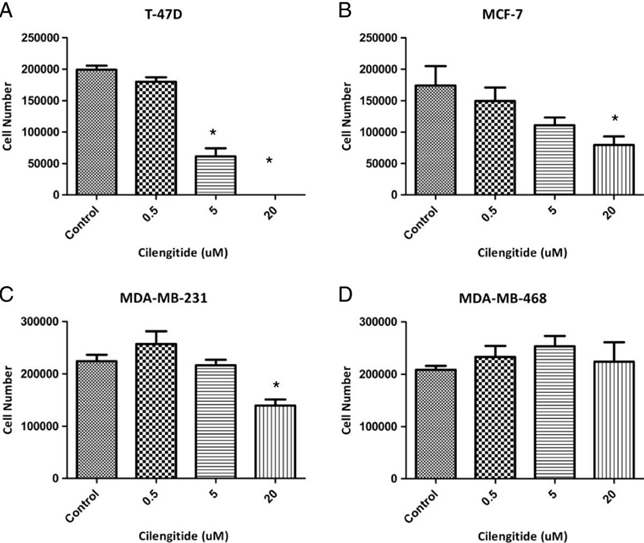
| MCF-7 | Apoptosis Assay | 0-20 μM | 48 h | induces apoptosis | 24153102 | |
| T-47D | Apoptosis Assay | 0-20 μM | 48 h | induces apoptosis | 24153102 | |
| MCF-7 | Growth Inhibition Assay | 0-20 μM | 96 h | inhibits cell growth in a dose dependent manner | 24153102 | |
| T-47D | Growth Inhibition Assay | 0-20 μM | 96 h | inhibits cell growth in a dose dependent manner | 24153102 | |
| FaDu | Apoptosis Assay | 25 µM | 48 h | induces apoptosis | 24557056 | |
| CAL27 | Apoptosis Assay | 25 µM | 48 h | induces apoptosis | 24557056 | |
| SCC25 | Apoptosis Assay | 25 µM | 48 h | induces apoptosis | 24557056 | |
| FaDu | Growth Inhibition Assay | 6.25–200 µM | 72 h | results moderate, dose-dependent growth inhibition | 24557056 | |
| CAL27 | Growth Inhibition Assay | 6.25–200 µM | 72 h | results moderate, dose-dependent growth inhibition | 24557056 | |
| SCC25 | Growth Inhibition Assay | 6.25–200 µM | 72 h | results moderate, dose-dependent growth inhibition | 24557056 | |
| H28 | Cell Viability Assay | 1 nM-200 μM | 72 h | decreases cell viability in a dose dependent manner | 24595274 | |
| MM05 | Cell Viability Assay | 1 nM-200 μM | 72 h | decreases cell viability in a dose dependent manner | 24595274 | |
| MSTO-211H | Cell Viability Assay | 1 nM-200 μM | 72 h | decreases cell viability in a dose dependent manner | 24595274 | |
| REN | Cell Viability Assay | 1 nM-200 μM | 72 h | decreases cell viability in a dose dependent manner | 24595274 | |
| LN-308 | Function Assay | 10 μm | 24 h | DMSO | reduces AhR protein levels and DRE reporter activity | 26500056 |
| HaCaT | Function Assay | 10 μm | 48 h | DMSO | reduces TGF-β2 mRNA expression | 26500056 |
| S-24 | Function Assay | 1/10 μm | 24 h | DMSO | reduces DRE reporter activity | 26500056 |
| ZH-161 | Function Assay | 1/10 μm | 24 h | DMSO | reduces DRE reporter activity | 26500056 |
| LN-308 | Function Assay | 1/10/100 μm | 24 h | DMSO | reduces DRE reporter activity in a concentration-dependent manner | 26500056 |
| M21 | Function assay | 1 hr | Binding affinity to integrin alphav/beta3 heterodimer in human M21 cells assessed as inhibition of integrin-mediated human M21 cell adhesion to vitronectin after 1 hr in presence of MnCl2, IC50 = 0.0004 μM. | 26753814 | ||
| HEK293T | Function assay | 2 hrs | Binding affinity to soluble truncated human recombinant Fc-tagged alphaVbeta3 and integrins were expressed in HEK293T cells after 2 hrs by competition ELISA-like assay, IC50 = 0.00051 μM. | 24095096 | ||
| HT-29 | Function assay | 2 hrs | Antagonist activity at integrin alphaVbeta5 (unknown origin) expressed in HT-29 cells assessed as reduction in cell adhesion to vitronectin after 2 hrs by MTT assay, IC50 = 0.12 μM. | 28351594 | ||
| HEK293 | Function assay | 2 hrs | Antagonist activity at integrin alphaVbeta3 (unknown origin) expressed in HEK293 cells assessed as reduction in cell adhesion to fibrinogen after 2 hrs by MTT assay, IC50 = 0.22 μM. | 28351594 | ||
| SKOV3 | Function assay | 2 hrs | Antagonist activity at integrin alphaVbeta3alphaVbeta5 (unknown origin) expressed in SKOV3 cells assessed as reduction in cell adhesion to fibrinogen after 2 hrs by MTT assay, IC50 = 0.37 μM. | 28351594 | ||
| U87MG | Function assay | 100 nM | 24 hrs | Inhibition of alphaVbeta3 integrin in human U87MG cells assessed as increase in p53 accumulation at 100 nM after 24 hrs by Western blot method | 29775303 | |
| U87MG | Function assay | 100 nM | 24 hrs | Inhibition of alphaVbeta3 integrin in human U87MG cells assessed as increase in p53 accumulation at 100 nM after 24 hrs in presence of MDM2 inhibitor nutlin-3 by Western blot method | 29775303 | |
| U87MG | Function assay | 100 nM | 24 hrs | Inhibition of alphaVbeta3 integrin in human U87MG cells assessed as increase in p53 accumulation at 100 nM after 24 hrs in presence of MDM2 inhibitor nutlin-3 and MDM4 inhibitor SJ-1722550 by Western blot method | 29775303 | |
| U87MG | Function assay | 100 nM | 8 hrs | Inhibition of alphaVbeta3 integrin in human U87MG cells assessed as increase in MDM2 mRNA expression at 100 nM after 8 hrs in presence of MDM2 inhibitor nutlin-3 and MDM4 inhibitor SJ-1722550 by RT-PCR method | 29775303 | |
| U87MG | Function assay | 100 nM | 24 hrs | Inhibition of alphaVbeta3 integrin in human U87MG cells assessed as increase in PUMA mRNA expression at 100 nM after 24 hrs by RT-PCR method | 29775303 | |
| U87MG | Function assay | 100 nM | 24 hrs | Inhibition of alphaVbeta3 integrin in human U87MG cells assessed as increase in PUMA mRNA expression at 100 nM after 24 hrs in presence of MDM2 inhibitor nutlin-3 by RT-PCR method | 29775303 | |
| U87MG | Function assay | 100 nM | 24 hrs | Inhibition of alphaVbeta3 integrin in human U87MG cells assessed as increase in PUMA mRNA expression at 100 nM after 24 hrs in presence of MDM4 inhibitor SJ-1722550 by RT-PCR method | 29775303 | |
| U87MG | Function assay | 100 nM | 24 hrs | Inhibition of alphaVbeta3 integrin in human U87MG cells assessed as increase in PUMA mRNA expression at 100 nM after 24 hrs in presence of MDM2 inhibitor nutlin-3 and MDM4 inhibitor SJ-1722550 by RT-PCR method | 29775303 | |
| U87MG | Function assay | 100 nM | 24 hrs | Inhibition of alphaVbeta3 integrin in human U87MG cells assessed as increase in p21 mRNA expression at 100 nM after 24 hrs by RT-PCR method | 29775303 | |
| U87MG | Antiproliferative assay | 100 nM | 72 hrs | Antiproliferative activity against human U87MG cells at 100 nM after 72 hrs in presence of MDM2 inhibitor nutlin-3 by MTS assay | 29775303 | |
| U87MG | Antiproliferative assay | 100 nM | 72 hrs | Antiproliferative activity against human U87MG cells at 100 nM after 72 hrs in presence of MDM2 inhibitor nutlin-3 and MDM4 inhibitor SJ-1722550 by MTS assay | 29775303 | |
| U87MG | Cell cycle assay | 100 nM | 24 hrs | Cell cycle arrest in human U87MG cells assessed as accumulation at G0/G1 phase at 100 nM after 24 hrs | 29775303 | |
| U87MG | Anti-invasive assay | 10 uM | 24 hrs | Anti-invasive activity in human U87MG cells at 10 uM after 24 hrs by transwell assay | 29775303 | |
| U87MG | Anti-invasive assay | 10 uM | 24 hrs | Anti-invasive activity in human U87MG cells at 10 uM after 24 hrs in presence of MDM2 inhibitor nutlin-3 and MDM4 inhibitor SJ-1722550 by transwell assay | 29775303 | |
| Click to View More Cell Line Experimental Data | ||||||
Chemical Information, Storage & Stability
| Molecular Weight | 702.68 | Formula | C29H41F3N8O9 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 199807-35-7 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | EMD 121974, NSC 707544 | Smiles | CC(C)C1C(=O)NC(C(=O)NCC(=O)NC(C(=O)NC(C(=O)N1C)CC2=CC=CC=C2)CC(=O)O)CCCN=C(N)N.C(=O)(C(F)(F)F)O | ||
Solubility
|
In vitro |
DMSO
: 100 mg/mL
(142.31 mM)
Water : 6.25 mg/mL Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such as vortex, ultrasound or hot water bath can be used to aid dissolving.
Mechanism of Action
| Targets/IC50/Ki |
αvβ3 receptor
(Cell-free assay) 4.1 nM
αvβ5 receptor
(Cell-free assay) 79 nM
|
|---|---|
| In vitro |
Cilengitide is a cyclized pentapeptide peptidomimetic designed to compete for the arginine-glycine-aspartic acid (RGD) peptide sequence that regulates integrin-ligand binding. Cilengitide selectively and potently blocks the ligation of the αvβ3 and αvβ5 integrins to provisional matrix proteins such as vitronectin, fibronectin, fibrinogen, von Willebrand factor, osteopontin, and others.
Cilegitide inhibits angiogenesis in vitro. 10 μM Cilengitide completely inhibits attachment of BAE, BME and HUVE cells on vitronectin and fibronectin. Cilengitide inhibits in vitro angiogenesis of BAE cells on three-dimensional collagen and fibrin gels pretreated with FGF-2(or VEGF-A) with IC50 of 15 μM and 8 μM, 4 μM and 3 μM, respectively.
Cilengitide blocks proliferation and induces apoptosis of endothelial cells as well as differentiation of human endothelial precursor cells (EPCs). 50 μg/mL Cilengitide completely inhibits the proliferation of human microvascular endothelial cell line HMEC-1 and leads to apoptosis in ~30% cells. 1.0 μM Cilengitide treating for 9 days inhibits the proliferation of EPCs by nearly 40%. 1 μM Cilengitide inhibits the differentiation of EPCs by more than 80% at 14 days.
Cilengitide inhibits adhesion and induces apoptosis of tumour cells. 25 μg/mL Cilengitide causes detachment of DAOY cells (medulloblastoma) and U87MG cells (glioblastoma) from vitronectin and tenascin by more than 60%. 25 μg/mL Cilengitide induces a nearly 50% apoptosis rate of these cells.
|
| Kinase Assay |
Integrin-binding competition assay
|
|
Recombinant soluble Integrins are immobilised, and peptides, which are serially diluted in Tris-buffered saline (TBS++) (0.1% (w/v) BSA, 150 mM NaCl, 1 mM CaCl2, 1 mM MgCl2 10 μM MnCl2, 20 mM Tris-HCl; pH 7.4), are added in parallel with biotinylated vitronectin (to 1μg/mL). After a 3-h incubation at 37℃ and washing with Tris–buffered saline, bound ligand is detected by incubation with an antibiotin alkaline phosphatase-conjugated antibody (BioRad) followed by development with p-nitrophenyl phosphatase substrate. The reaction is stopped by the addition of NaOH and the colour intensity read at 405 nm.
|
|
| In vivo |
Cilengitide is active against tumour growth and angiogenesis as a single-agent. 100 μg Cilengitide induces a significant decrease in the number of CD31+ vessels seen in tumours (2/high-power field) compared with control tumours (56/high-power field). 100 μg Cilengitide increases cellular apoptosis in the brain tumours of animals (2.2% apoptotic cells/high-power field) compared with those receiving the inactive peptide (1.7% cells/high-power field). Cilengitide treatment results in prolonged survival of the mice bearing melanoma xenografts M21 compared with control treatment group. (36.5 vs 17.3 days).
Cilengitide can augment the therapeutic benefit associated with cytotoxic agents including chemotherapy and radiation therapy in tumour models. Cilengitide (250 mg/dose) alone does not alter tumour growth of breast cancer xenografts when compared with untreated mice, but combined modality RIT (CMRIT) using RIT and six doses of Cilengitide (250 mg/dose) increases efficacy of treatment, with the cure rate for mice that receives only RIT increasing from 15 to 53%. CMRIT significantly increases apoptosis of tumour and endothelial cells 5 days, and decreases tumour proliferation.
|
References |
|
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
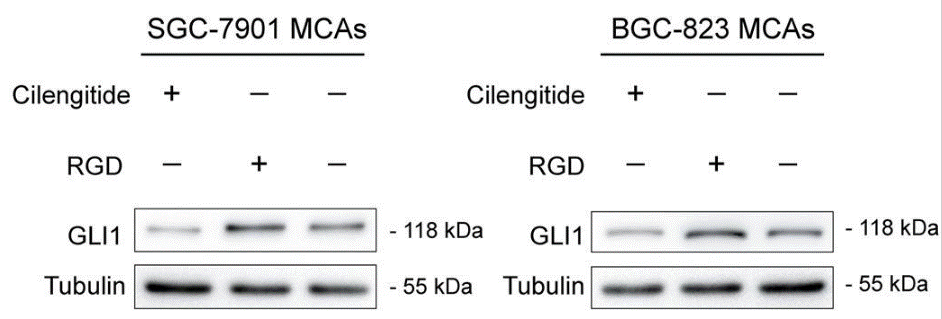
| Western blot | GLI1 pFAK / p-AKT |
|
31366904 |
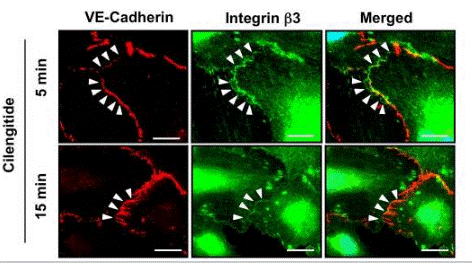
| Immunofluorescence | VE-cadherin / β3 integrin |
|
19212436 |
| Growth inhibition assay | Cell number |
|
24153102 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT01517776 | Terminated | Gliomas |
Martin-Luther-Universität Halle-Wittenberg|Merck KGaA Darmstadt Germany |
January 2012 | Phase 2 |
| NCT01118676 | Completed | Locally Advanced Non Small Cell Lung Cancer (NSCLC) |
Institut Claudius Regaud|Merck KGaA Darmstadt Germany |
March 2010 | Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.
Frequently Asked Questions
Question 1:
The recommended vehicle for it is 30% propylene glycol, 5% Tween 80, 65% D5W at 30mg/ml. Can you let me know if this is a suspension or clear solution?
Answer:
S7077 can be dissolved in 30% propylene glycol/5% Tween 80/65% D5W at 10 mg/ml as a clear solution.
Question 2:
Is it a TFA salt?
Answer:
S7077 is actually a TFA salt, and the ratio between it and TFA is 1:1.
