Technical Data
| Formula | C20H28O2 |
||||||||||
| Molecular Weight | 300.4 | CAS No. | 302-79-4 | ||||||||
| Solubility (25°C)* | In vitro | DMSO | 60 mg/mL (199.73 mM) | ||||||||
| Ethanol | 4 mg/mL (13.31 mM) | ||||||||||
| Water | Insoluble | ||||||||||
| In Vivo (Add solvents to the product individually and in order.) |
|
||||||||||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
|||||||||||
Preparing Stock Solutions
Biological Activity
| Description | Retinoic acid (Tretinoin, Vitamin A acid) is a ligand for both the retinoic acid receptor (RAR) and the retinoid X receptor (RXR), and can induce granulocytic differentiation and apoptosis in acute promyelocytic leukaemia (APL) cells. Tretinoin (NSC 122758) can be used to induce animal models of osteoporosis. | ||
|---|---|---|---|
| Targets |
|
||
| In vitro | Retinoic acid (Tretinoin, Vitamin A acid) prevents the cytotoxicity of H2O2 in cultured human mesangial cells by increasing both the catalase activity and the reduced glutathione content, which are dose- and time-dependent changes. Its incubation leads to increased levels of catalase mRNA and gamma-glutamyl-cysteine synthetase (catalytic subunit) mRNA, the latter being the rate-limiting step in de novo reduced glutathione synthesis. This compound also upregulates matrix metalloproteinase-13 and matrix metalloproteinase-8 in human keloid-derived fibroblasts. |
||
| In Vivo | Retinoic acid (Tretinoin, Vitamin A acid) has been shown to reduce renal cortex protein content by 30% in treated old male Fischer 344 rats compared to controls, an effect potentially mediated through inhibition of tumor necrosis factor-beta1 and osteopontin expression. It also prevents major steroid-induced biomechanical changes in hairless mouse dermal connective tissue that contribute to atrophy. When combined with imiquimod, this compound leads to tattoo fading and moderate pigment clearance on histopathology in guinea pigs. Additionally, it increases fibroblastic proliferation in skin incisions made in CD-1 mice, though collagen production is diminished. |
Protocol (from reference)
| Cell Assay: |
|
|---|---|
| Animal Study: |
|
References
|
Customer Product Validation
-
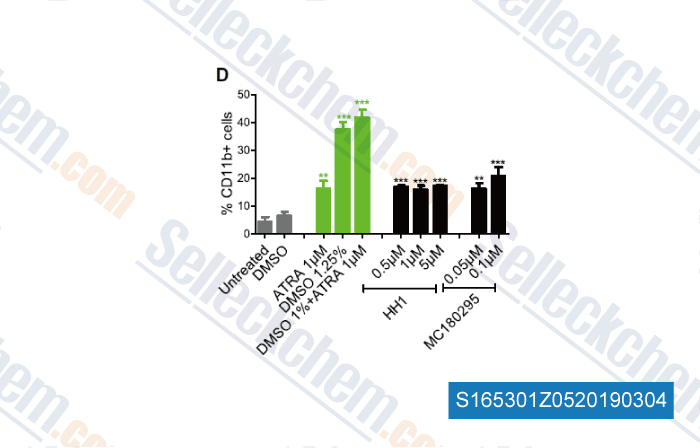
Data from [ , , Cell, 2018, 175(5):1244-1258 ]
Sellecks Retinoic acid (Tretinoin, Vitamin A acid) Has Been Cited by 40 Publications
| CPSF6-RARγ interacts with histone deacetylase 3 to promote myeloid transformation in RARG-fusion acute myeloid leukemia [ Nat Commun, 2025, 16(1):616] | PubMed: 39805830 |
| LDH nanoparticles-doped cellulose nanofiber scaffolds with aligned microchannels direct high-efficiency neural regeneration and organized neural circuit remodeling through RhoA/Rock/Myosin II pathway [ Biomaterials, 2025, 314:122873] | PubMed: 39369670 |
| Construction of human pluripotent stem cell-derived testicular organoids and their use as humanized testis models for evaluating the effects of semaglutide [ Theranostics, 2025, 15(6):2597-2623] | PubMed: 39990223 |
| Extracellular lactate improves neurogenesis by modulating H3K9 lactylation and SnoN expression under hypoxic conditions [ Stem Cell Res Ther, 2025, 16(1):462] | PubMed: 40866997 |
| Human epicardial organoids from pluripotent stem cells resemble fetal stage with potential cardiomyocyte- transdifferentiation [ Cell Biosci, 2025, 15(1):4] | PubMed: 39825425 |
| Study of Retinoic Acid-Induced Osteoarthritis: Integrating RNA-Sequencing, Network Pharmacology, Molecular Docking, and Experimental Validation [ Int J Mol Sci, 2025, 26(12)5519] | PubMed: 40564983 |
| Potassium Iodide Induces Apoptosis in Salivary Gland Cancer Cells [ Int J Mol Sci, 2025, 26(11)5199] | PubMed: 40508009 |
| Modelling myocardial ischemia/reperfusion injury with inflammatory response in human ventricular cardiac organoids [ Cell Prolif, 2024, e13762.] | PubMed: 39377453 |
| Patient-derived rhabdomyosarcoma cells recapitulate the genetic and transcriptomic landscapes of primary tumors [ iScience, 2024, 27(10):110862] | PubMed: 39319271 |
| The Effect of Retinoic Acid on Arsenite-Transformed Malignant UROtsa Bladder Cancer Cells: In Vitro Model of Basal Muscle-Invasive Bladder Cancer [ Cancers (Basel), 2024, 16(6)1178] | PubMed: 38539513 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
