Technical Data
| Formula | C27H27N5O2 |
||||||
| Molecular Weight | 453.54 | CAS No. | 405554-55-4 | ||||
| Solubility (25°C)* | In vitro | DMSO | 46 mg/mL (101.42 mM) | ||||
| Water | Insoluble | ||||||
| Ethanol | Insoluble | ||||||
| In Vivo (Add solvents to the product individually and in order.) |
|
||||||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
|||||||
Preparing Stock Solutions
Biological Activity
| Description | SB590885 is a potent B-Raf inhibitor with Ki of 0.16 nM in a cell-free assay, 11-fold greater selectivity for B-Raf over c-Raf, no inhibition to other human kinases. | ||
|---|---|---|---|
| Targets |
|
||
| In vitro | SB590885 displays significant selectivity for B-Raf over c-Raf with Ki of 0.16 nM over 1.72 nM. This compound is a more potent inhibitor than the previously described Raf/VEGFR kinase inhibitor BAY 439006 (Ki = 38 nM for mutant B-Raf, 6 nM for c-Raf). It displays potent selectivity over 46 other kinases. Unlike the multi-kinase inhibitor BAY43-9006, this chemical stabilizes the oncogenic B-Raf kinase domain in an active configuration. In Colo205, HT29, A375P, SKMEL28, and MALME-3M cells expressing oncogenic B-RafV600E, this compound treatment potently inhibits ERK phosphorylation with EC50 of 28 nM, 58 nM, 290 nM, 58 nM, and 190 nM, respectively, and consistently, inhibits the proliferation with EC50 of 0.1 μM, 0.87 μM, 0.37 μM, 0.12 μM, and 0.15 μM, respectively. It decreases anchorage-independent growth of melanoma cell lines in a BRAF mutant-selective manner. This chemical displays high affinity for B-Raf with Kd of 0.3 nM. Most of the melanoma cell lines that harbour the BRAF V600E mutation and lack CDK4 mutations (451Lu, WM35, and WM983) are highly sensitive to this compound with IC50 of <1 μM. Increased levels of cyclin D1 resulting from genomic amplification mediate this compound resistance in B-Raf V600E-mutated melanomas. | ||
| In Vivo | Administration of SB590885 potently decreases tumorigenesis in murine xenografts established from mutant B-Raf-expressing A375P melanoma cells, and modestly inhibits tumour growth. | ||
| Features | Displays significant selectivity for B-Raf over c-Raf. |
Protocol (from reference)
| Cell Assay:[1] |
|
|---|---|
| Animal Study:[1] |
|
References
|
Customer Product Validation
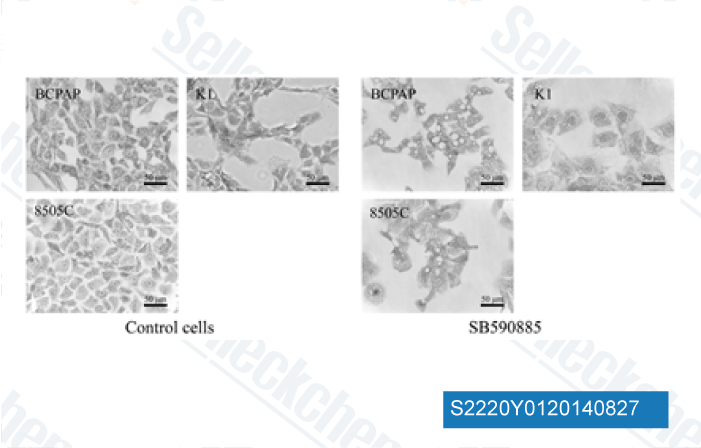
-
Data from [ Invest New Drugs , 2014 , 32(4), 626-35 ]
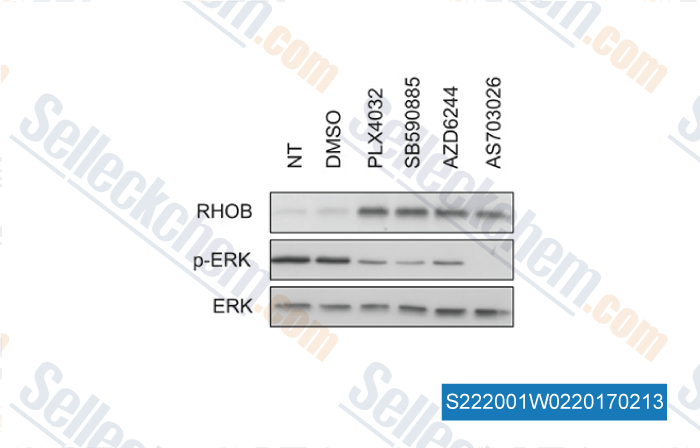
-
Data from [ , , Oncotarget, 2015, 6(17):15250-64. ]
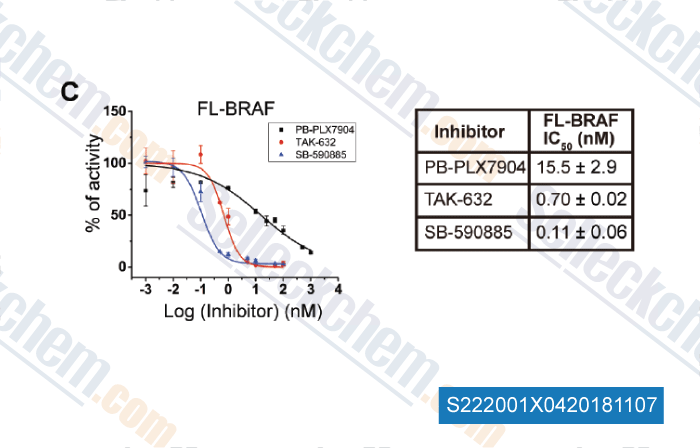
-
Data from [ , , Chembiochem, 2018, doi:10.1002/cbic.201800359 ]
Sellecks SB590885 Has Been Cited by 42 Publications
| NLRP7 maintains the genomic stability during early human embryogenesis via mediating alternative splicing [ Commun Biol, 2025, 8(1):125] | PubMed: 39865169 |
| Self-renewing human naïve pluripotent stem cells dedifferentiate in 3D culture and form blastoids spontaneously [ Nat Commun, 2024, 15(1):668] | PubMed: 38253551 |
| A transient transcriptional activation governs unpolarized-to-polarized morphogenesis during embryo implantation [ Mol Cell, 2024, 84(14):2665-2681.e13] | PubMed: 38955180 |
| Chromatin landscape dynamics during reprogramming towards human naïve and primed pluripotency reveals the divergent function of PRDM1 isoforms [ Cell Death Discov, 2024, 10(1):474] | PubMed: 39562537 |
| Integrated drug response prediction models pinpoint repurposed drugs with effectiveness against rhabdomyosarcoma [ PLoS One, 2024, 19(1):e0295629] | PubMed: 38277404 |
| Generation of a humanized mesonephros in pigs from induced pluripotent stem cells via embryo complementation [ Cell Stem Cell, 2023, 30(9):1235-1245.e6] | PubMed: 37683604 |
| Modeling human pregastrulation development by 3D culture of blastoids generated from primed-to-naïve transitioning intermediates [ Protein Cell, 2023, 14(5):337-349] | PubMed: 37155315 |
| Short C-terminal Musashi-1 proteins regulate pluripotency states in embryonic stem cells [ Cell Rep, 2023, 42(10):113308] | PubMed: 37858462 |
| Chemical-induced epigenome resetting for regeneration program activation in human cells [ Cell Rep, 2023, 42(6):112547] | PubMed: 37224020 |
| Discordance between chromatin accessibility and transcriptional activity during the human primed-to-naïve pluripotency transition process [ Cell Regen, 2023, 10.1186/s13619-023-00179-2] | PubMed: 37938437 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
